
It’s a generally accepted statistic that up to 90 percent of startups fail. So it’s not surprising that some of the promising companies reported on over the past 20 years are no longer in existence.
But maybe it’s even more surprising how many of the startups you enjoyed reading about are still in business — some of them succeeding spectacularly.
So… where are they now?
We answered that question by tracking 21 companies established between 1990 and 2012. We did not include household names such as Mobileye (started in 1999) and Waze (established in 2009) — we all know what happened to these success stories.
ARGO MEDICAL TECHNOLOGIES

Background: Argo Medical Technologies, founded in 2001, commercialized the ReWalk exoskeleton that enables paraplegics to walk — and even do marathons.
Past coverage: In July 2008, ISRAEL21c broke the news about ReWalk’s first clinical trials, creating a storm of interest in the company.
What happened since then: Argo morphed into ReWalk Robotics, which went public in 2014 and still is based in Yokne’am in northern Israel, with offices in Berlin and Massachusetts.
The company invented two solutions for individuals with lower limb disabilities: The ReWalk Personal 6.0 Exoskeleton for spinal cord injury (FDA approved in 2014; CE approved in select countries) and ReStore Exo-Suit for stroke rehabilitation (CE and FDA approval in 2019).
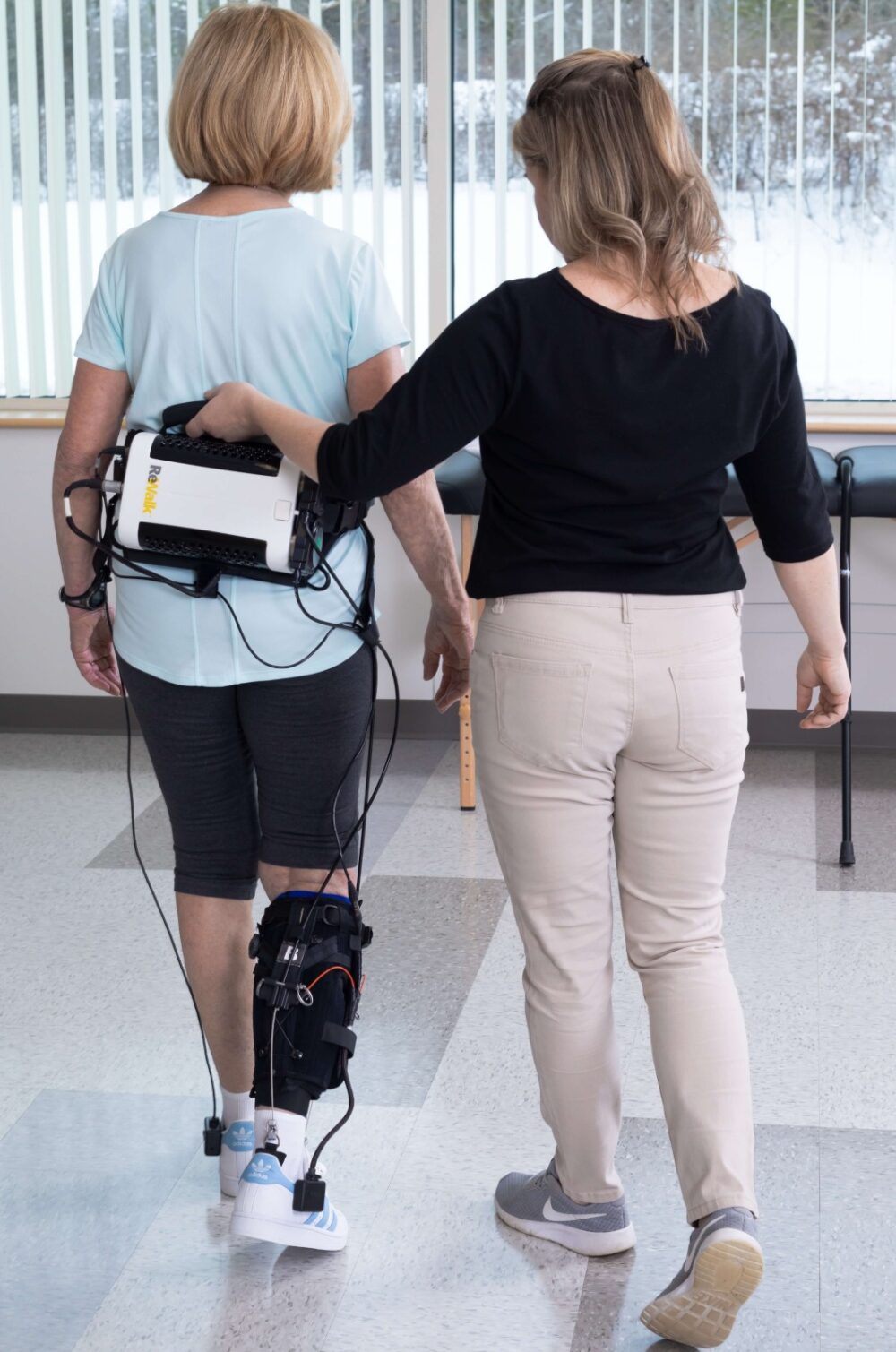
ReBoot, a lightweight, battery-powered orthotic exo-suit for home use, recently received FDA Breakthrough Device designation on its way to market.
Argo founder Amit Goffer, a quadriplegic, and other ReWalk colleagues founded UPnRIDE Robotics in 2013 to develop a wheeled robotic device providing upright and seated mobility for anyone with difficulty standing or walking.
UPnRIDE received FDA clearance just before the pandemic. Some were sold in Israel and several more are being tested at the Veterans Affairs Medical Center the Bronx, New York. The company is raising funds to begin international marketing and develop the next model.
BETTER PLACE
Background: Better Place launched in 2007 with a bold idea for charging electric cars: Instead of plugging in for half an hour to get just 80 miles of range (the state-of-the-art in 2007), drivers could swap their spent battery in under five minutes at robot-operated “switching stations” across Israel.
Past coverage: In October 2007, ISRAEL21c reported on CEO Shai Agassi’s “daring” Project Better Place.
What happened since then: The company raised $850 million and built stunning visitor centers in Israel and China, but ultimately sold only about 1,000 of its compatible cars in Israel and a few hundred in Denmark. The company declared bankruptcy in 2013.
CTO Barak Hershkovitz joined General Motors. Mike Granoff, who headed up oil independence policies and government outreach for Better Place, became a venture capitalist focusing on mobility. Agassi tried starting another electric vehicle company in London and now spends his time investing, speaking and advising other companies.
The concept of battery swapping lives on in at least two other startups: Ample in the U.S. and NIO in China.
BRAINSWAY
Background: BrainsWay developed a TMS (transcranial magnetic stimulation) helmet outfitted with an electromagnetic energy-emitting coil that, when worn for 15 minutes a day, stimulates the brain to alleviate a variety of ailments. BrainsWay ran its first tests in 2005 on a single Alzheimer’s patient but the first medical application was for chronic depression.
Past coverage: This article in February 2006 told of a “magnetic remedy for depression.”
What happened since then: BrainsWay received European CE approval to treat autism, Alzheimer’s, bipolar disorder, chronic pain, major depressive disorder, Parkinson’s, schizophrenia, smoking cessation, post-traumatic stress disorder, multiple sclerosis, OCD and stroke rehabilitation.
The U.S. FDA has authorized BrainsWay for the treatment of drug-resistant depression; in 2015, the U.S. Navy bought Brainsway helmets to improve healthcare for service personnel and their families.
After building up the company to 70 people, former CEO Uzi Sofer has since moved on and now heads up Alpha Tau, which uses alpha radiation to treat solid tumors.
CARTIHEAL
Background: CartiHeal was founded in 2009 to develop and commercialize the world’s first safe and effective off-the-shelf cartilage regeneration solution.
Past coverage: This article, published in January 2013, prompted a flood of reader inquiries.
What happened since then: CartiHeal’s revolutionary Agili-C implant for the treatment of cartilage lesions in arthritic and non-arthritic joints was granted CE approval for the European Union in 2012 and Breakthrough Device Designation by the U.S. Food and Drug Administration in 2020. A premarket approval application is now under FDA review.
In August, two-year results of a multinational, randomized, controlled IDE (investigational device exemption) clinical study on 251 patients with knee-joint injuries or defects demonstrated Agili-C’s superiority over the current surgical standard of care.
The promising trial results prompted U.S.-based Bioventus, which invested $15 million in 2020, to initiate acquisition of CartiHeal, to be finalized after FDA approval (expected in the second half of 2022). The company, based in Kfar Saba with about 30 employees, will continue manufacturing Agili-C in Israel after the acquisition, according to CEO-founder Nir Altschuler.
CUPRON
Background: In the early 2000s, Cupron developed an embedded copper-based antimicrobial, antifungal technology for healthcare, consumer, industrial and military fabrics including medical textiles and facemasks. In the first field test of Cupron’s technology, the Chilean miners trapped in the Copiapó mine were provided with prototypes of Cupron’s antifungal socks in 2010.
Past coverage: The first of several ISRAEL21c stories about Cupron appeared in February 2007.
What happened since then: Cupron initially focused on European and Israeli markets but in the past eight years shifted to the U.S. healthcare market and established a headquarters, warehouse and R&D lab in Richmond, Virginia.
More than 25 U.S. hospitals use Cupron fabrics for anything that touches patients, including linens, pajamas and towels, to reduce hospital-acquired infections. Cupron also licenses its technology to makers of socks, underwear, seating and cosmetic pillowcases, among other items.
The private company also retains a headquarters in Herzliya to serve the Israeli market, with customers such as Hogla-Kimberly adult diapers for sensitive skin; Perrigo diabetic socks; Delta anti-athletes’ foot socks; MedCu wound dressings; and Netafim antifungal irrigation drippers.
DEEPBREEZE
Background: “Seventy percent of the patients who go to a general practitioner have some kind of complaint about their respiratory tract, whether that’s flu, bronchitis, asthma or poor breathing,” DeepBreeze founder Dr. Igal Kushnir told ISRAEL21c in 2006. DeepBreeze pioneered the use of VRI — vibration response imaging — which has the potential to replace MRIs, ultrasounds and even the stethoscope.
Pressure sensors are attached to the patient’s back to pick up lung vibrations, which are used to generate a dynamic image of the air flow into the lung. The whole process takes just five minutes. By 2006, the company was selling its VRIxp flagship product in Europe. In 2007, the company entered a partnership with GE Healthcare.
Past coverage: In October 2006, ISRAEL21c explained DeepBreeze’s “non-invasive pulmonary imaging technology designed to help clinicians diagnose, assess and treat various respiratory conditions.”
What happened since then: The company expanded its product line in 2011 to monitor lungs in COPD patients using the Breeze@Home system. However, despite raising nearly $14 million, the company closed its doors in 2013.
DeepBreeze CEO Miki Nagler has served as VP of advanced technologies for LandaLabs ever since then, focusing on nanotechnology for drug delivery, nano-metallics and alternative energy. Kushnir still works as a physician in Israel and heads up MitralCord, which addresses mitral valve regurgitation, one of the primary types of heart disease.
GROW FISH ANYWHERE
Background: Founded in 2008 based on an invention by Hebrew University Prof. Jaap van Rijn, GFA (Grow Fish Anywhere) developed and commercialized a fully closed, zero-discharge aquaculture system for raising freshwater and saltwater fish. The environmentally friendly system can operate in any climate without nearby sources of water.
Past coverage: The first of several ISRAEL21c articles about GFA appeared in December 2010.
What happened since then: GFA was acquired by Local Ocean, which used the Israeli technology to establish a large fish plant in upstate New York in 2017. Local Ocean folded two years later.
Some of the original GFA founders launched Pure Blue Fish in 2016, an Israeli company with an American subsidiary in Virginia.
A new spinoff company, Ever Blue, combines GFA technology with a new development from van Rijn’s lab to neutralize an “off taste” in the fish due to compounds produced in the closed system. “The aquaculture business as a whole is going forward fast,” van Rijn tells ISRAEL21c.
ICECURE MEDICAL
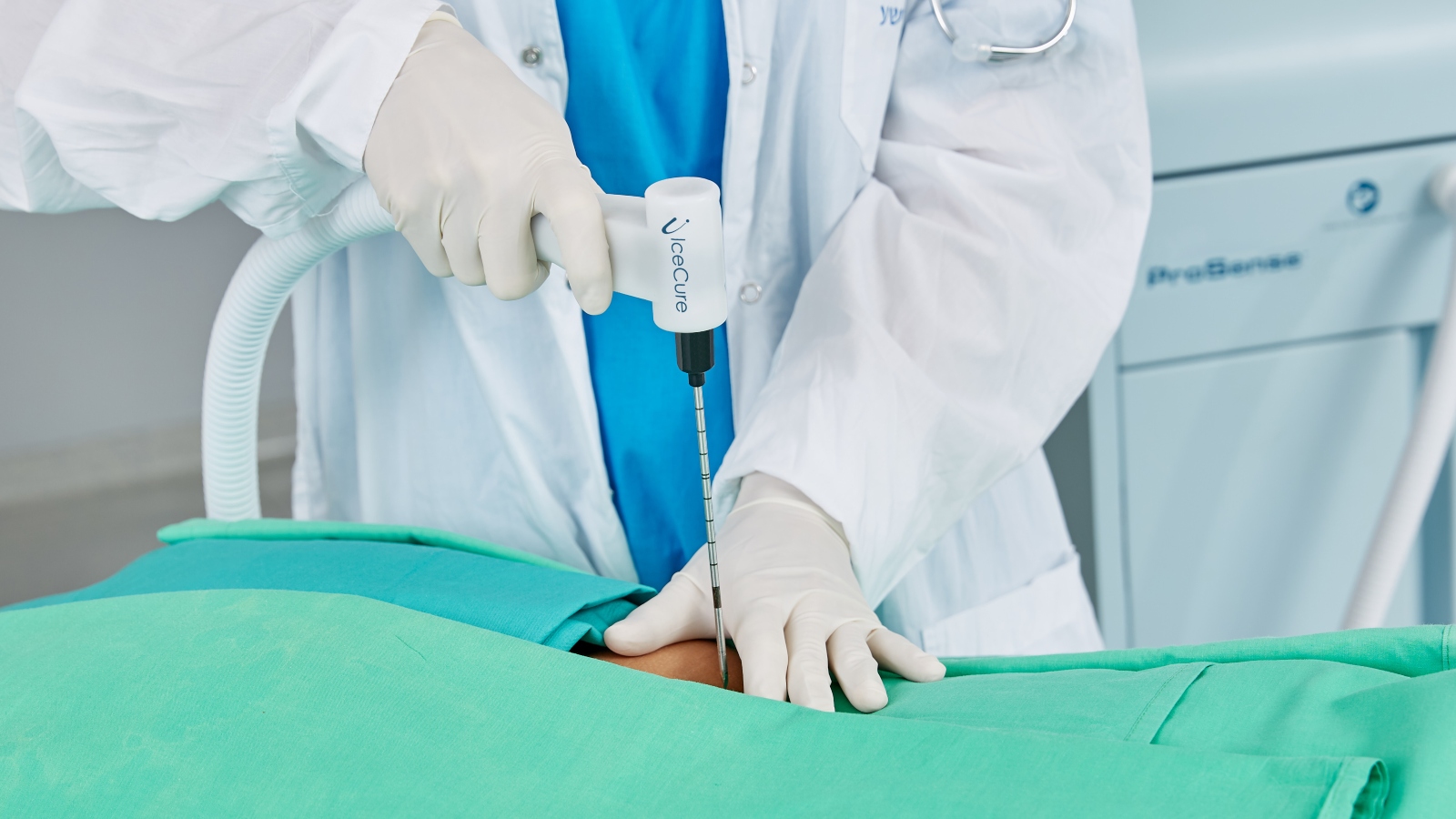
Background: Founded in 2006, Caesarea-based IceCure Medical developed a minimally invasive cryoablation technique to destroy tumors by freezing them with liquid nitrogen. The procedure, guided by ultrasound or CT imaging under local anesthesia, takes five to 15 minutes in the doctor’s office.
Past coverage: In July 2012, ISRAEL21c reported that IceCure’s novel device was poised to revolutionize how American doctors remove benign breast lumps.
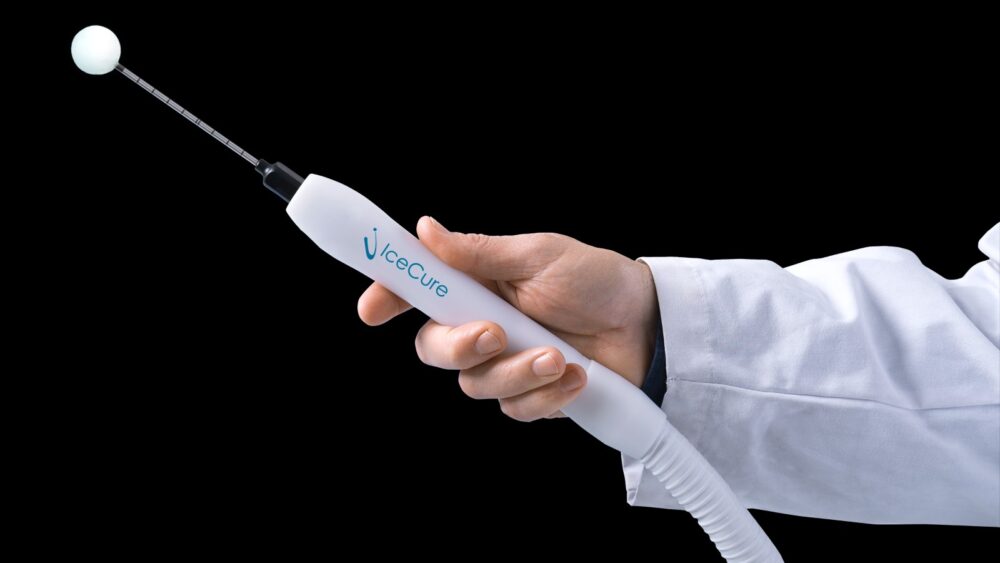
What happened since then: IceCure’s ProSense system is marketed and sold worldwide for the treatment of stage 1 or 2 breast, kidney, bone and lung tumors — the applications so far approved by the U.S. FDA and European CE. ProSense got FDA “breakthrough device” designation in April for treating patients with T1 invasive breast cancer and/or breast cancer that cannot be treated surgically.
Interim data from the company’s ICE3 clinical trial on cryoablation of small, low-risk breast cancer was shared at the recent Radiologists Society of North America meeting. In that multiyear trial at 19 American hospitals, only four of 194 patients experienced cancer recurrence over three years. They will be monitored for a total of five years.
On December 13, IceCure announced that it raised $17 million in an underwritten public offering, to be used toward new product development.
INSIGHTEC
Background: InsighTec’s Exablate is a noninvasive, MRI-guided ultrasound treatment that thermally ablates (destroys) tumors without an incision. It was introduced in 1999 to address uterine fibroids and later was expanded to cover bone cancer. TIME magazine named InsighTec’s MR Guided Focused Ultrasound (MrgFUS) one of the 50 best inventions of 2011.
Past coverage: This article in January 2008 introduced Exablate 2000 for bone cancer.
What happened since then: The company has pivoted to focus on its newer Exablate Neuro focused ultrasound product to treat motor symptoms of Parkinson’s disease. Using ultrasound in the brain is considered revolutionary because it can pass the blood-brain barrier, which drugs cannot.
Exablate Neuro received FDA approval for this indication in November 2021, having previously received FDA clearance in 2016 to address essential tremors and in 2018 to treat tremor-dominant Parkinson’s. Treatment often requires just a single session.
Some 37 U.S. medical centers are using Exablate Neuro. Future indications may include epilepsy, OCD, neuropathic pain and depression. The company is headquartered in Haifa and now has offices in Dallas, Miami, Shanghai and Tokyo.
KAMADA
Background: Kamada was founded in 1990 as a biopharmaceutical company specializing in plasma-derived therapeutics. Headquartered in Rehovot with a manufacturing facility in Beersheva, Kamada went public on the Tel Aviv Stock Exchange in 2005 and on NASDAQ in 2013.
Past coverage: In February 2005, ISRAEL21c reported that Kamada’s treatment for congenital emphysema was granted orphan status by the FDA.
What happened since then: Kamada’s two main globally commercialized products are Glassia for a certain type of emphysema; and Kedrab, a human rabies immunoglobulin.
In November, Kamada acquired four FDA-approved plasma-derived hyperimmune products from Saol Therapeutics for underserved or unserved patient populations, such as organ transplant recipients. Manufacturing is expected to be transferred to Beersheva.
In 2020, Kamada began clinical trials of a plasma-derived hyperimmune immunoglobulin therapy for COVID-19. Those trials are continuing.
In 2021, the company established Kamada Plasma, a wholly owned U.S.-based plasma collection company, and began commercial operations in the United States.
MAGSHOE
Background: When Richard Reid attempted to detonate a bomb hidden in his shoe on a December 2001 flight from Paris to Miami, it ushered in 20 years of security protocols at airports where travelers were required to remove their shoes.
IDO Securities’ MagShoe aimed to do away with this uncomfortable and time-consuming requirement with a special metal detector. Travelers would step on the MagShoe and, in just over a second, its sensors alert security inspectors to objects as small as a knife blade hidden in their shoes.
Past coverage: This article in 2004 described MagShoe as the only technology of its kind.
What happened since then: After it was first released in 2004, the MagShoe was tested at airports in Israel, Italy, Spain, Poland and other European countries. But by 2013, IDO Security was gone, along with its flagship product. IDO’s trading value had already sunk into the penny stocks category by 2012 and a lawsuit over spam policies at the company cut the share price in half.
Today, improvements in airport security technology have allowed some airports to do away with the requirement to remove one’s shoes, reducing the need for a once innovative product.
MEDIWOUND
Background: Founded in 2000, MediWound is a biopharmaceutical company that uses an enzymatic technology platform to make novel biotherapeutic solutions for burn and wound care and tissue repair.
Past coverage: This article from September 2002 told readers about MediWound’s new surgery-free burn therapy.
What happened since then: Today, MediWound’s NexoBrid pineapple-based treatment for severe thermal burns is marketed in the European Union and other countries, and soon in the United States.
MediWound was the first Israeli biotech company to form a drug commercialization partnership with an Emirati corporation when it signed a distribution agreement for NexoBrid with the UAE’s Ghassan Aboud Group in December 2020.
Additional products in development are EscharEx, a bioactive topical therapy for debridement of chronic and hard-to-heal wounds; and MW005, a topical biological drug for treating non-melanoma skin cancers. Ongoing Phase 2 studies on patients with diabetic foot ulcers and venous leg ulcers indicate that a few daily applications of EscharEx are well-tolerated, safe and effective.
MODU
Background: Dov Moran’s startup, M-Systems, made the disk-on-key (or thumb drive) a household word. When M-Systems was acquired by SanDisk in 2006 for $1.6 billion, Moran turned to his next innovation: Modu, which set out to create the world’s smallest smartphone with “jackets” you could slip on and off to give it a new look and new functionality – a music player, a messaging device, a game platform.
Past coverage: In February 2008, Modu’s tiny modular cellphone seemed poised for greatness.
What happened since then: Modu didn’t stand a chance against Apple’s iPhone, released the year before Modu’s debut. The company’s intellectual property and patents were acquired by Google for just $4.9 million.
Moran reportedly lost $15 million on Modu, but he went on to found or invest in dozens of companies through Grove Ventures, the $100 million fund he established in 2017. These include Consumer Physics (which makes SCiO, a portable material analysis sensor for the agriculture industry), Sensible Medical Innovation (wearable devices to measure lung fluid), GlucoMe (a digital diabetes care platform), Wiliot (smart sensors for tracking products), Kidoz (a safe mobile network for kids) and TriEye (sensors for autonomous vehicles). In addition, more than 30 companies have been started by ex-Modu staffers.
MOOVIT
Background: Moovit started life in 2012 as Tranzmate, the “Waze for public transportation.” Waze cofounder Uri Levine was its first chairman of the board and key investor. The app allows commuters to find the best route to their destination by bus, train or tram. It started with a few hundred thousand users in six cities.
Past coverage: ISRAEL21c first wrote about Moovit in 2013.
What happened since then: Moovit now boasts more than one billion users in 3,400 cities across 112 countries in 45 languages.
In May 2020, Intel acquired Moovit for more than $1 billion with plans to integrate the company’s tech into Intel’s other big transportation play in Israel, Mobileye. Moovit data is critical to improving the navigation abilities of Mobileye’s self-driving cars. Moovit eventually will also work as the interface for hailing a Mobileye robo-taxi.
Moovit recently launched a carpool function in collaboration with Waze; a partnership with “smart cane” company WeWalk to help visually impaired individuals navigate public transport; and integrations with electric scooter companies Bird and Lime.
PAULEE CLEANTEC
Background: Paulee CleanTec got started in 2008 after entrepreneur Oded Halperin was fined for not picking up after his dog, Paulee. Halperin enlisted serial inventor Prof. Oded Shoseyov to find a greener solution for dog waste. He devised a low-cost chemical process that converts droppings into a sterile, odor-free organic mineral fertilizer in minutes.
Past coverage: In October 2011, this article described how Paulee CleanTec planned to turn its patented technology into a unique pooper-scooper for the American pet-supply market.
What happened since then: Although the pooper-scooper hasn’t yet found the right commercial partner, Paulee forged ahead with other projects.
Its U.S. affiliate, Epic CleanTec, collects residential building wastewater, applies Paulee technology to transform the solid waste to fertilizer for landscaping, and recycles the remaining water for non-potable uses. Established in 2014, Epic has set up three installations in California and more are planned.
Its Brazilian affiliate, Ludologic, creates five powdered agricultural fertilizers — targeted to sugarcane, soy, oranges, coffee and corn — from waste sludge. Next: fertilizer for forestry. In 2022, Ludologic will build a large-scale production line in Sao Paulo to generate 100 tons of fertilizer per day.
In Israel, Paulee has implemented its BioLizer system for livestock farms. The system converts half a ton of fresh animal manure into odorless organic fertilizer in one hour. The pandemic held up plans to expand to Europe and the U.S., but this is expected soon.
POWERMAT
Background: With the introduction of MagSafe for the iPhone, wireless charging technology is being positioned as the next big thing. But back in 2006, when Powermat launched, charging a device without plugging it into a cable was pure science fiction.
The Powermat solution consists of a mat that’s plugged into the wall and a removable “puck” that’s attached to your device. Within a short time of its founding, Powermat had inked a $5 million deal with General Motors to put wireless chargers in the carmaker’s Cadillacs and Chevys (with an aim to eventually charge the entire vehicle wirelessly) and another one with Procter & Gamble’s Duracell battery brand.
Past coverage: ISRAEL21c first wrote about Powermat in January 2008.
What happened since then: Powermat signed additional partnerships, including with Starbucks, Coffee Bean & Tea Leaf, AT&T, Samsung, LG and Harman and several McDonald’s restaurants.
In 2018, Powermat and the Alliance for Wireless Power announced an initiative to create a standard for wireless charging, dubbed AirFuel. The group includes Israeli wireless charging startup Humavox.
“We understood that for this revolution to take hold, we would have to create an entire ecosystem,” said then-Powermat CEO Ran Poliakine, who now heads early cervical-cancer detection startup Illumigyn.
PRONEURON BIOTECHNOLOGIES
Background: World-renowned neuroimmunologist Michal Schwartz from the Weizmann Institute of Science founded Proneuron Biotechnologies in 1996 to develop treatments using macrophages (a type of immune system cell) for spinal-cord injury and paralysis.
Past coverage: This profile of Schwartz appeared in August 2003.
What happened since then: Once Schwartz understood the mechanism for bringing macrophages to the brain, she realized the immune system could be harnessed to counter the effects of neurodegenerative diseases. She won a patent to use Copaxone, a drug that reacts with immune cells, to research this possibility. Copaxone, invented at the Weizmann, had been licensed to Teva Pharmaceuticals for treating multiple sclerosis.
Although Teva agreed to test Copaxone in Parkinson’s and Alzheimer’s patients for Proneuron, that never happened. A 14-year legal battle ensued. In 2020, the courts ruled in favor of Proneuron developing Copaxone on its own for treating neurodegenerative diseases. But the ongoing lawsuit had already bankrupted Proneuron.
Schwartz founded a new company, ImmunoBrain Checkpoint, in 2015 to develop novel neuroprotective immune therapies. She tells ISRAEL21c that the first-in-human clinical trial is planned for early this year.
PYTHAGORAS SOLAR
Background: Gonen Fink was one of the first employees of Check Point Software Technologies. After 12 years, he left Check Point in 2007 to found Pythagoras Solar with Itay Baruchi. They intended to make power-generating, energy-efficient transparent photovoltaic windows.
Past coverage: This article from August 2011 described how the window could be retrofitted into existing office blocks to replace energy-seeping glass windows — a process that would pay itself back within five years.
What happened since then: Although Pythagoras won international attention and awards including the GE Ecomagination Challenge, the technology and the market weren’t quite ready.
Fink put the window on the back burner, dissolved the company in 2013 and joined LightCyber, an Israeli cybersecurity company, which was acquired by Palo Alto Networks. Today, Fink is an adviser and investor in the cyber industry.
SEAMBIOTIC
Background: Seambiotic, founded in 2003, introduced a revolutionary technology to channel smokestack carbon-dioxide emissions through cleansing pools of algae. These algae could then be used as a basis for biofuels or nutraceuticals.
Past coverage: August 2007 was the first of many times ISRAEL21c reported on Seambiotic’s approach to capturing carbon and producing climate-positive products.
What happened since then: Seambiotic set up a pilot plant with the Israel Electric Corporation in Ashkelon and started pursuing business deals in the United States, Italy and China.
“The idea was great, but unfortunately the performance was not as good as the idea,” former Seambiotic VP of Business Development Noam Menczel tells ISRAEL21c. “The major obstacle we could not overcome was getting FDA approval. The company went out of business in 2015.”
TRANSPHARMA MEDICAL
Background: An estimated 20 percent of people around the world suffer from trypanophobia, a fear of needles. TransPharma Medical’s ViaDerm drug-delivery system used a painless preloaded patch instead of a needle. It was originally created to help patients suffering from severe osteoporosis, who previously required a daily injection in a clinic or hospital. TransPharma’s patch could be applied at home using a disposable microelectrode array and a reusable handheld electronic control unit.
Past coverage: In August 2006, this article promised “goodbye to needles.”
Status today: In 2008, TransPharma entered into a licensing agreement with pharma giant Eli Lilly. But a trial of the technology failed, and Eli Lilly terminated the agreement, which had been TransPharma’s main source of income. In 2012, the company’s assets were acquired by Syneron Medical for $3.6 million.
Under its new owners, the promise of TransPharma for osteoporosis was jettisoned and ViaDerm has been repositioned to deliver cosmetic substances through the skin.
URBAN AERONAUTICS

Background: Ever since TV’s George Jetson commuted to work in a flying car, the prospect of taking to the air in one’s own personal hovering vehicle has captivated science fiction writers and consumers alike. Urban Aeronautics has been working toward that dream since 2002.
Its initial product was the X-Hawk, a helicopter with internal rotors, making it safer to land in a crowded urban environment. Next up was the Cormorant UAV, which served as the basis for the company’s main product today, the CityHawk VTOL (“vertical take-off and landing”) vehicle.
Past coverage: In September 2002, ISRAEL21c reprinted an article from Globes about Urban Aeuronautics’ X-Hawk.
What happened since then: CityHawk won’t be ready to take consumers on their commutes until close to the end of the decade. In the meantime, Urban Aeronautics signed a deal in 2020 with New York-based Emergency Medical Services provider Hatzolah Air to bring the City Hawk to EMS applications.
While an unmanned Cormorant could carry up to 1,400 pounds of cargo, the CityHawk is being designed to transport five passengers and a pilot. Unlike other flying cars in development, CityHawk will use hydrogen fuel cells rather than electric batteries. Hydrogen can be refueled in minutes and hydrogen tanks are lighter, too — important where every pound can be critical to range and operation.
Produced in association with ISRAEL21c.
Recommended from our partners
The post Where Are They Now? Startups That Succeeded Or Failed Over The Last 20 Years appeared first on Zenger News.




